|
公司基本資料信息
|
|||||||||||||||||||||||||||||
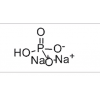
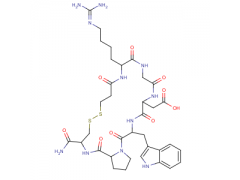
Synonym: INTEGRELIN;EPTIFIBATIDE ACETATE;MAP-LYS-GLY-ASP-TRP-PRO-CYS-NH2;Mpr-Harg-Gly-Asp-Trp-Pro-Cys-NH2,( Disulfide Bridge:1-7);Eptifibatide;Human Eptifibatide;Eptifitide;Mpr-Harg-Gly-Asp-Trp-Pro-Cys-NH2,( Disulfide Bridg on Mpr and Cys)
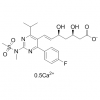
MF: C35H49N11O9S2
MW: 831.96
Product Category: proteins;Amino Acid Derivatives;Peptide
Pharmacological Effects: Eptifibatide is a platelet glycoprotein Ⅱb/Ⅲa receptor reversible antagonist,adverse reactions are mild , it can be discontinued immediately while adverse reactions occur . It has Strong effect and high selectivity . It has no antigenicity,and it does not cause allergic reactions. It is used For acute coronary syndrome, coronary intervention before treatment and acute Q-wave myocardial infarction. It can relieve unstable angina symptoms and reduce the incidence of cardiovascular events. It can limit the non-Q-wave myocardial infarction,and reduce through wall myocardial infarction occurrance.
Eptifibatide is used to treat acute coronary syndrome, the starting amount is 180μg/kg, the intravenous maintenance dose is per minute 2μg/kg continuous intravenous infusion 72h, if implementing the primary percutaneous coronary intervention, continuous intravenous drip after surgery is 18~24h. For percutaneous coronary intervention, the starting amount is 180μg/kg intravenous injection, after 2μg/kg continuous intravenous infusion every minute, after 10 min, administrated again with 180μg/kg intravenously per minute , thereafter 2μg/kg continuous intravenous infusion for 18~24h.
Eptifibatide is a cyclic heptapeptide, by preventing fibrinogen, von Willebrand factor and other adhesive ligands binding to GPIIb/IIIa , it can reversibly inhibit platelet aggregation. When administrated intravenously, vitro inhibition of platelet aggregation of eptifibatide is in a dose-and concentration-dependent manner. Inhibition of platelet aggregation is reversible after Eptifibatide infusion is stopped , which is believed to be caused by dissociation of eptifibatide and platelet.
Platelet membrane glycoprotein (GP) Ⅱb/Ⅲa receptor antagonists: after platelet activation, platelet membrane GP Ⅱb/Ⅲa receptor changes its conformation to bind to the end of fibrinogen dimer to complete platelet aggregation. So, GP Ⅱb/Ⅲa receptor is believed to be the final common pathway of platelet aggregation. platelet GP Ⅱb/Ⅲa receptor antagonist Currently in clinical use, it has the following three types; ① abciximab (Abciximab reopro) is the Fab fragment of a monoclonal antibody of platelet GP Ⅱb/Ⅲa receptor. ②Eptifibatide, Integrilin is a cyclic heptapeptide. ③ tirofiban is a small molecule non-peptide compound.
Eptifibatide for injection is a small molecule with heptapeptide containing Lys-Gly-Asp amino acid sequence (KGD), which is like fibrinogen recognizing and binding site GPⅡb/Ⅲa receptors ,it can highly and specially bind to platelets GPⅡb/Ⅲa receptors, it is the GPⅡb/Ⅲa receptor specific competitive inhibitor ,with a low affinity, high dissociation rate, short plasma half-life , platelet aggregation restores baseline levels within 4-8 hours after stopping the infusion . American College of Cardiology (ACC) and the American Heart Association (AHA) guidelines recommend eptifibatide injection can be used for acute coronary syndrome (unstable angina, non-ST-segment elevation myocardial infarction) or supporting through percutaneous coronary intervention (PCI) drug treatment , it is continuous used for 72 hours.
Long-term animal studies on the carcinogenic potential of eptifibatide are not made. the Ames test, mouse lymphoma cells (L 5178Y, TK +/-) forward mutation test, the human lymphocyte chromosome aberration assay, or mouse micronucleus test, have shown that eptifibatide is not genotoxic. continuous infusion for a daily dose of the Eptifibatide reaching 72 mg/kg/day (calculated according to body surface area, it is 4 times of the maximum recommended human daily dose), has no effect on fertility and reproductive capacity of male and female rats.



 通過(guò)認(rèn)證
通過(guò)認(rèn)證