|
公司基本資料信息
|
|||||||||||||||||||||||||||||
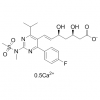
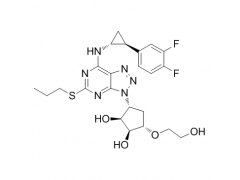
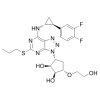
Synonym: 1,2-Cyclopentanediol, 3-[7-[[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3H-1,2,3-triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)-, (1S,2S,3R,5S)-;Ticagrelor, >=98%;Ticagrelo;Ticargrelor;Brilinta;Possia;Ticagrelor and intermediates;Ticagrelor Standards
MF: C23H28F2N6O4S
MW: 522.57
Product Category: AZD6140;Ticagrelor;Cardiovascular APIs;Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;Sulfur & Selenium Compounds
Boiling Point: 777.6±70.0 °C(Predicted)
Density: 1.67
pKa: 13.26±0.70(Predicted)
Indications and Usage: Ticagrelor, a new platelet aggregation inhibitor successfully developed by AstraZeneca (U.S.), is the world’s first reversible combination orally-administered P2Y12 adenosine diphosphate receptor antagonist.
It is used to reduce cardiovascular death and heart attacks in patients with acute coronary syndrome (ACS.) Rapid onset after oral administration, and can effectively improve symptoms of patients with ACS. Thienopyridines is a reversible P2Y12 inhibitor, so it is particularly applicable towards patients who need to undergo anticoagulant therapy before surgery.
Description: In December 2010, the P2Y12 receptor antagonist ticagrelor (also known as AZD6140) was approved in Europe for the treatment of acute coronary syndrome (ACS), a condition that covers several clinical symptoms with the potential to cause acute myocardial ischemia (MI). ADP binds to two purinergic receptors, the P2Y1 and P2Y12 receptors. The action of ADP binding to the P2Y12 receptor results in activation of the GP Ⅱb/Ⅲa (integrin) receptor.GP Ⅱb/Ⅲa initiates and prolongs platelet aggregation, which in turn results in the cross-linking of platelets through fibrin and finally thrombus formation. Inhibition of ADP stimulation of the P2Y12 receptor has been found to be an effective strategy for managing the atherothrombotic events associated with ACS and potentially resulting from percutaneous coronary intervention (PCI, stent implantation) .
Chemical Property: White solid
Uses: Ticagrelor, the first reversible oral P2Y12 receptor antagonist, provides faster, greater, and more consistent ADP-receptor inhibition than Clopidogrel. Used in the treatment of acute coronary syndromes (ACS)
Ticagrelor is the first reversibly binding oral P2Y12 receptor antagonist, also inhibits CYP2C9 and 4-hydroxylation with IC50 of 10.5 μM and 8.2 μM respectively
Clinical Use: Ticagrelor, discovered and developed by AstraZeneca, is a platelet adenosine diphosphate (ADP) P2Y12 (P2T) reversible receptor antagonist approved in the E.U. in 2010 and launched in Germany and the UK in 2011 for the treatment of patients with acute coronary syndromes (ACS). It was approved in the U.S. and Canada in 2011 following successful clinical trial results in patients with ACS which showed it to be superior to preexisting drugs for reducing death due to vascular causes. Ticagrelor is an oral drug indicated for use in combination with acetylsalicylic acid (aspirin) for the prevention of atherothrombotic events in adult patients with ACS (unstable angina, non-ST elevation myocardial infarction (NSTEMI), or ST elevation myocardial infarction (STEMI)). Unlike its competitors prasugrel and clopidogrel, which require bioactivation, ticagrelor is not a prodrug and does not require in vivo activation. It has a rapid onset of action, relatively rapid reversibility, greater potency, and exhibits consistency in platelet inhibition. Following dosing, ticagrelor reaches Cmax in about 1.5 h, with formation of a major metabolite with equipotent intrinsic activity to the parent compound.



 通過認(rèn)證
通過認(rèn)證